Periodic Trends are essential concepts in chemistry that describe patterns in the properties of elements as you move across the periodic table. These trends arise from the arrangement of elements based on their atomic number. The properties of atoms follow predictable patterns across the periodic table and are crucial for understanding the behavior of atoms in chemical reactions.
A Mantra for understanding in Chemistry.
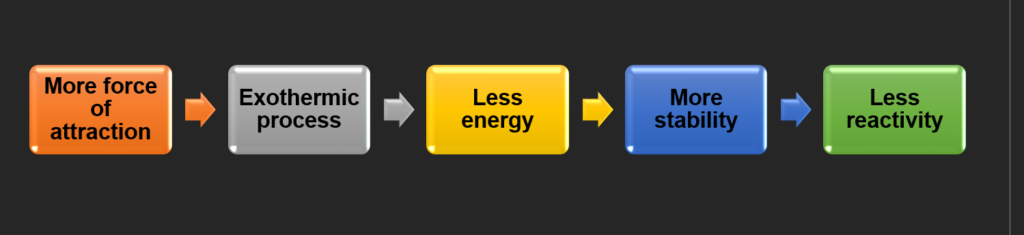
When atoms or molecules experience a Greater Force of Attraction, they tend to move towards a more stable state. As they do so, the system releases energy, often in the form of heat, making it a Exothermic Process. This release of energy leads to a Lower Overall Energy state for the system, which corresponds to Greater Stability. A more stable system will have Lower Reactivity because it is already in a low-energy, favorable condition. As a result, substances that form strong attractions typically have lower reactivity, as they have little incentive to undergo further chemical changes.
Coulomb’s Law-
According to Coulomb’s Law, the magnitude of the force (FFF) between two point charges is directly proportional to the product of the magnitudes of the charges (q1 and q2) and inversely proportional to the square of the distance (r) between them. It can be expressed as :
Coulomb’s law can be used for qualitative comparison of attractions among the charged particles in molecules, ions and within the atom etc. Here q1 and q2 are the charges on the particles and r is the distance of separation between the charged particles.
Qualitative Meaning of Coulomb’s law
- Force of attraction is directly proportional to the product of the charges on the particles. It means more charges means more force of attraction and more energy released due to that attraction.
- Force of attraction is inversely proportional to the distance of separation between the charged particles. More distance of separation, more far away charged particles from each other means less attraction means less energy released due to that attraction.
Force of attraction ∞ Energy(more energy released)
Energy is negative because more attraction means more exothermic as energy decreases with increase in attraction. According to sign convention, exothermic energy or energy released is negative energy.
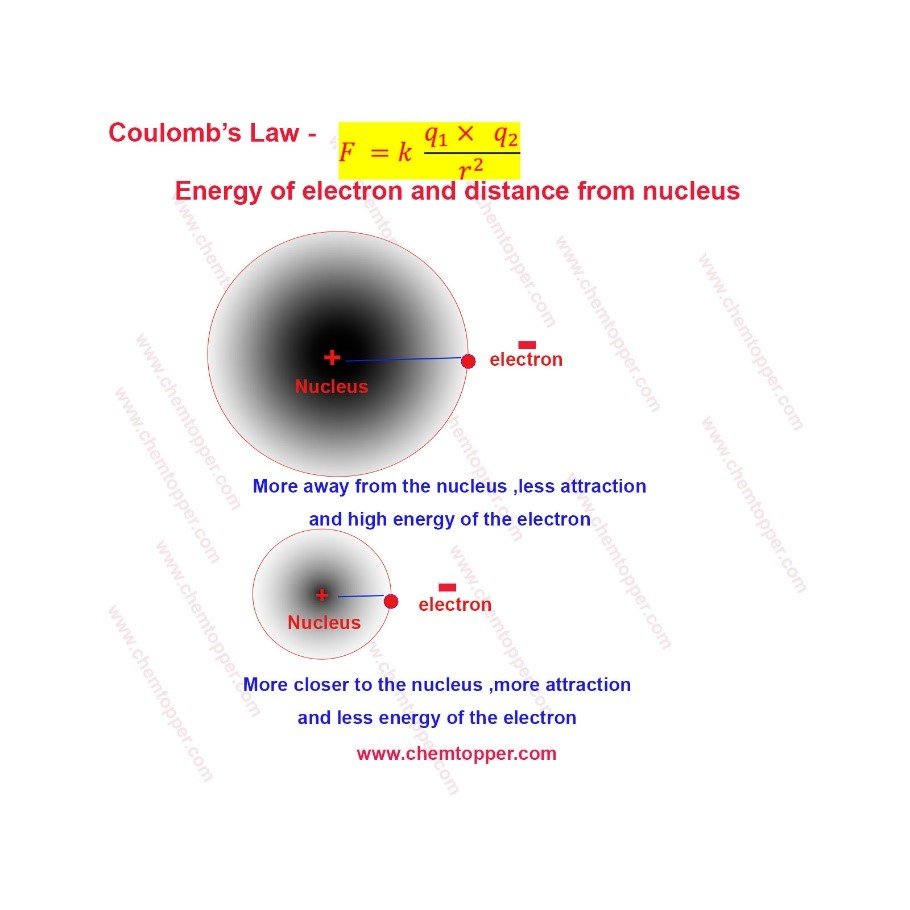
Energy of electron based on Coulomb’s law
More is the distance between the electron and nucleus, less is the attraction and more is the energy of the electron.
Less is the distance between the electron and the nucleus, more is the attraction and less is the energy of the electron.
One more example of Coulomb’s Law – Force of attractions of ions with water (polar molecule)
Energy of the Electron in an Orbital
Energy of the electron in an orbital depends on a number of factors. These are enumerated below:
1. Distance of the orbitals from the nucleus.
It’s clear from the above picture as the shell number is increasing, distance of the electron from the nucleus increases and hence energy of the electron is increasing as attraction is decreasing.
2. Screening or Shielding Effect
Screening or shielding effect is due to shielding of nuclear charge by inner shell electrons.
More are the number of inner shell electrons; more is the nuclear charge screening and lesser the positive charge felt by valence electrons.
For example, Na has 10 electrons screening its outermost shell electrons and potassium has 18 electrons screening its outermost shell. So, screening effect is more on valence electron of potassium then sodium.
Na -[Ne] 3s1
K -[Ar] 4s1
It means valence electron of potassium is less tightly bound to the nucleus due to more screening of nuclear charge and less attraction with the nucleus.
The presence of shielding electrons reduces the electrostatic attraction between the positively charged protons in the nucleus and the outer electrons. Moreover, the repulsive forces between electrons in a many-electron atom further offset the attractive force exerted by the nucleus.
3. Penetration Effects Of The Orbitals
Penetration effects of different orbitals can be understood from the probability distribution curves of the electrons in these orbitals. It has been observed that penetration effect follows this order-
More is the penetration effect of the orbitals, more closer is the electron to nucleus which means more attraction between the electron and the nucleus and less energy.
Notice in above picture that the 2p orbital has its maximum probability closer to the nucleus than for the 2s. This might lead us to predict that the 2p would be preferable (lower energy) to the 2s orbital. However, notice the small hump of electron density that occurs in the 2s profile very near the nucleus. This means that although an electron in the 2s orbital spends most of its time a little farther from the nucleus than does an electron in the 2p orbital, it spends a small but very significant amount of time very near the nucleus. We can say that the 2s electron penetrates to the nucleus more than once in the 2p orbital. This penetration effect causes an electron in a 2s orbital to be attracted to the nucleus more strongly than an electron in a 2p orbital. That is, the 2s orbital is lower in energy than the 2p orbitals in a polyelectronic atom.
From above electron distribution picture, it is can be decided that penetration effect of different orbitals are
3s> 3p> 3d
Greater penetration means more closer to the nucleus and stronger attraction of the electron with the nucleus which means less energy of the electron.
In general, the more effectively an orbital allows its electron to penetrate the shielding electrons to be close to the nuclear charge, the lower is the energy of that orbital. Since the stability of an electron is determined by the strength of its attraction to the nucleus.








