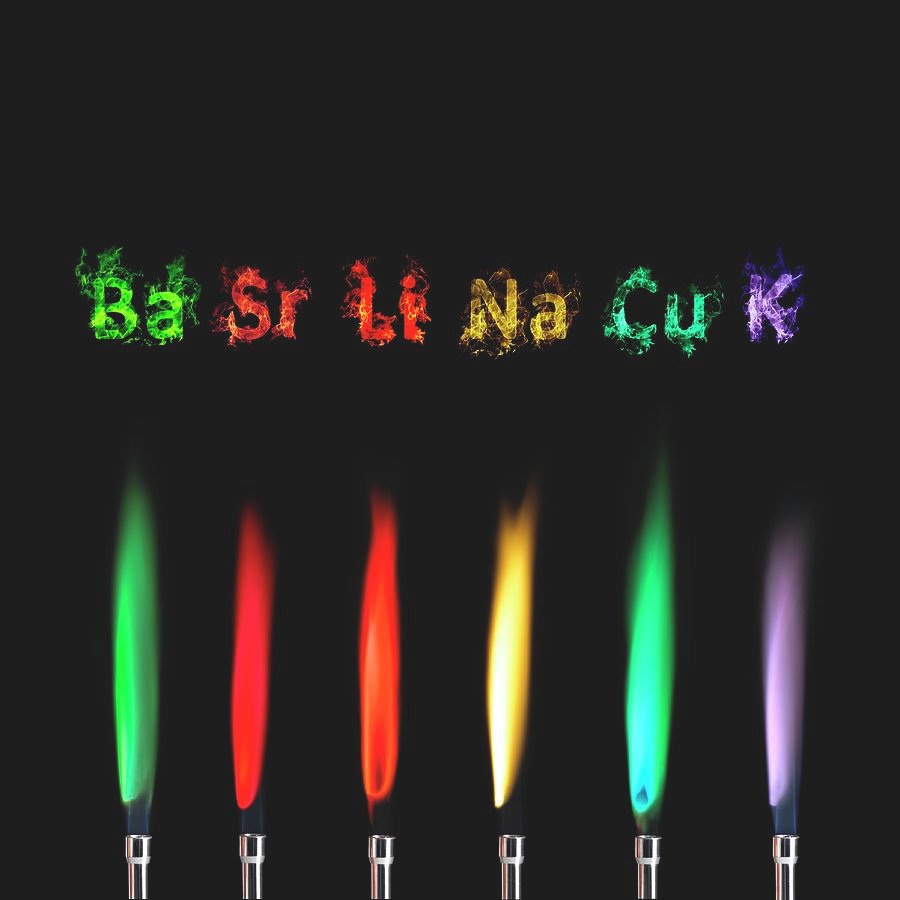By Mehr Grewal
What is a flame test and how does it work? Today, I will give you an overview of this common experiment that you might do in your chemistry class.
To understand the flame test, you need to know about the electromagnetic spectrum.
Light has a dual nature; it is made up of both particles and waves. This was proved by Thomas Young’s double-slit experiment that he performed in 1801. If you’re still confused, think of it this way: if you visit a lake or a pond near your home and throw a stone in the water, you will see ripples emanating from the spot where you threw the stone. These ripples are small waves. But what is water fundamentally made out of? If you were to zoom in really, really closely on a drop of water with an electron microscope, you would see that water is made out of H2O molecules. You could say that water is made up out of both particles and waves. Just like that, light is made up of really, really small particles called photons.
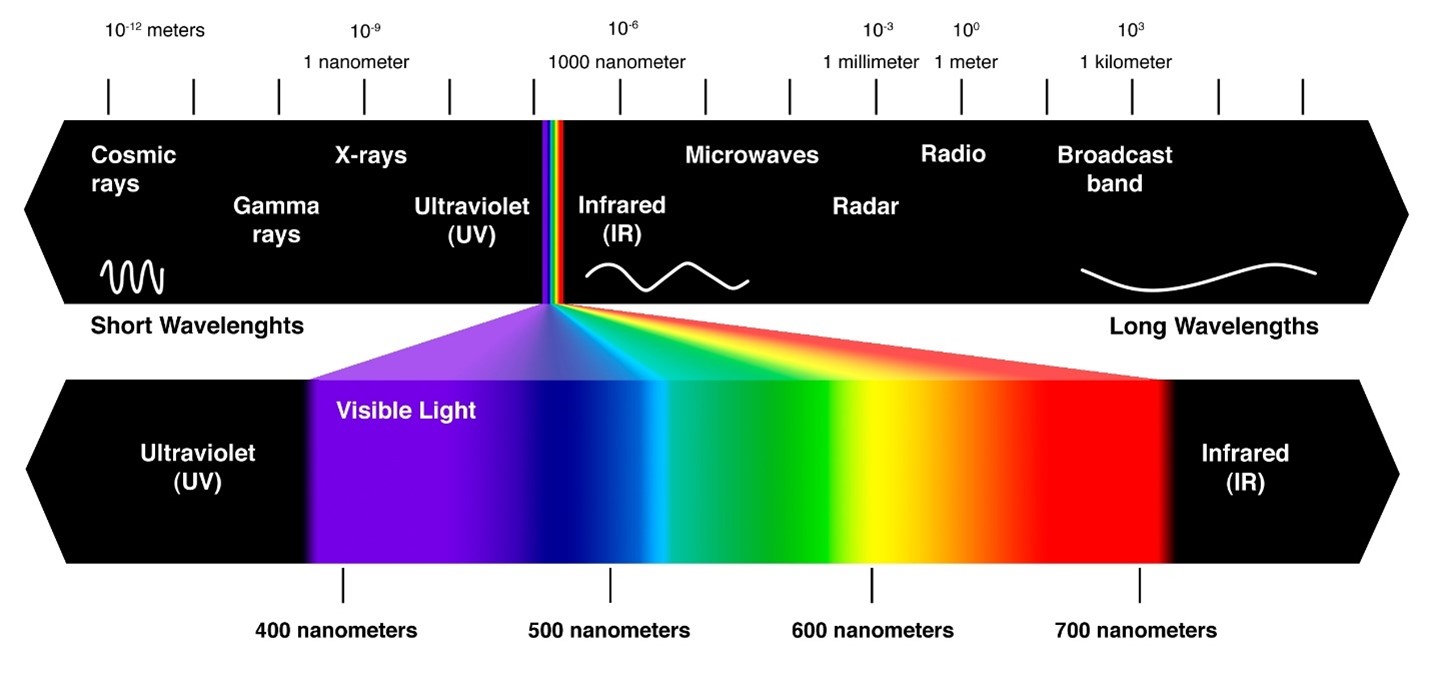
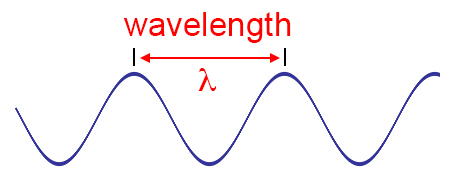
The colors and light that we see around us are really energy or electromagnetic radiation. And they are only a small portion – visible light – of the whole electromagnetic spectrum. The colors/energy that you see around you are in the form of waves. And waves have different frequencies, or energies. Frequency is defined as waves per second, or how many waves pass through a given point in a second. Waves also have different wavelengths or the distance from a point on one wave to the corresponding point on the next wave.
The higher the frequency of a part of the electromagnetic spectrum, the lower the wavelength. Colors at the end of the rainbow like blue and violet have higher frequencies than colors like red and orange. If you were to go higher than blue or violet, you would be out of the range of visible light into ultraviolet (UV) light, X-Rays, and gamma rays. You can’t see any of those forms of energy, but they can be harmful if you are exposed to them too much.
If you went lower than red, you would have infrared light, radio waves, and microwaves. Radio waves are used for communication, because they have such long wavelengths and can travel long distances. Below is a picture of the electromagnetic spectrum where you can see the different forms of electromagnetic radiation.
Now that we’ve talked about electromagnetic radiation, let’s talk about the flame test. The essential principle of the flame test is that when electrons inside an atom are energized, for example, through heat, they get “excited,” and jump energy levels into an “excited state,” and then jump back down to their ground state. When they do so, the energy they absorbed is released, causing them to display a characteristic emission spectrum, which you can see in the form of the flame changing color.
When you do this experiment, you will perform it using compounds that contain metals, for example, sodium nitrate, lithium chloride, potassium chloride, etc. This is because in the case of metals, it’s easier to excite the electrons and cause the process described above to occur.
You will see that each element changes the flame to a distinctive color. You may have to see the flame color change through a cobalt glass, which filters out the yellow light characteristic of sodium. Why do we want to filter out this light? Because sodium is a common contaminant in many of these compounds and we want to get a clearer picture. It also helps us to differentiate between colors that might appear similar, so that we can better identify the compound.