🍬 How Do We Count Really Tiny Things?
In everyday life, we often count things in groups. For example:
- 👟 A pair of shoes means 2 shoes
- 🍌 A dozen bananas means 12 bananas
These group words—like pair or dozen—can be used for anything. You can have a pair of cats 🐱 (2 cats) or a dozen eggs 🥚 (12 eggs).
Now imagine you are selling jelly beans 🍬, and someone wants 50 or even 500 of them. Wouldn’t it take forever to count each one?
💡 Here’s a Smart Trick:
- Count 10 jelly beans.
- Weigh them on a scale ⚖️.
Let’s say the total weight of 10 jelly beans is:
![]()
Now calculate the average weight of one jelly bean:
![]()
🔄 Use Weight to Count Faster!
If someone asks for 50 jelly beans:
![]()
✅ Just weigh out 55 grams instead of counting!
What if someone wants 500 jelly beans? Just multiply:
![]()
🎯 It’s that easy to “count” using weight!
🔬 What About Atoms?
Atoms are super tiny—so small we can’t see or count them one by one.
But just like we used the mass of jelly beans to count them, scientists use the mass of atoms to count atoms too!
Instead of counting billions of atoms, we just measure their mass. This is the exact same idea used in chemistry, especially when working with moles and Avogadro’s number.
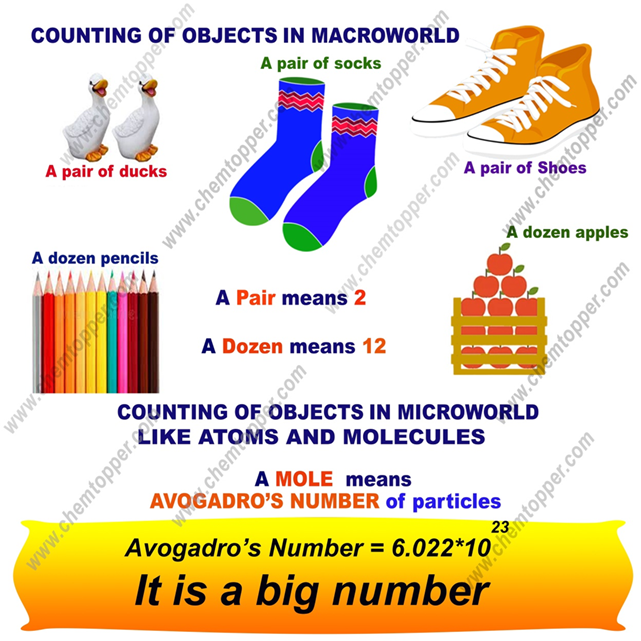
🧠 What is Avogadro’s Number?
Avogadro’s number (NA) is the number of atoms in 12 grams of carbon-12.
![]()
This means:
![]()
😮 How Big is This Number?
Avogadro’s number is incredibly large! If every person on Earth started counting atoms at 1 atom per second, it would take over 3 million years to finish counting the atoms in just 1 mole!
📊 What Do These Numbers in the Periodic Table Mean?
The atomic masses in the periodic table are actually the mass of 6.022 × 10²³ atoms of that element.
![]()
🔄 How to Convert Between Grams and Atoms
We use Avogadro’s number like a bridge to convert:
- Atoms → Grams
- Grams → Atoms
📘 Example 1: How many atoms in 24 g of carbon?
We know:
![]()
Use the ratio:
![]()
📘 Example 2: How many grams in 6.022 × 10²⁴ atoms of carbon?
![]()
📘 Example 3: What is the mass of one mole of oxygen atoms?
Given:
![]()
Then:
![]()
✅ Matches the atomic mass of oxygen in the periodic table.
🎯 Summary
- Avogadro’s number helps us count particles using mass.
- It connects grams ↔ atoms using a fixed ratio.
- One mole = 6.022 × 10²³ particles.
💡 Chemistry made simple with Avogadro!