Counting of Macroscopic Particles like apples, socks etc. can be done using small numbers.Macroscopic particles are big and visible so they can be counted easily.
Counting of microscopic particle like atoms and molecules need big numbers.
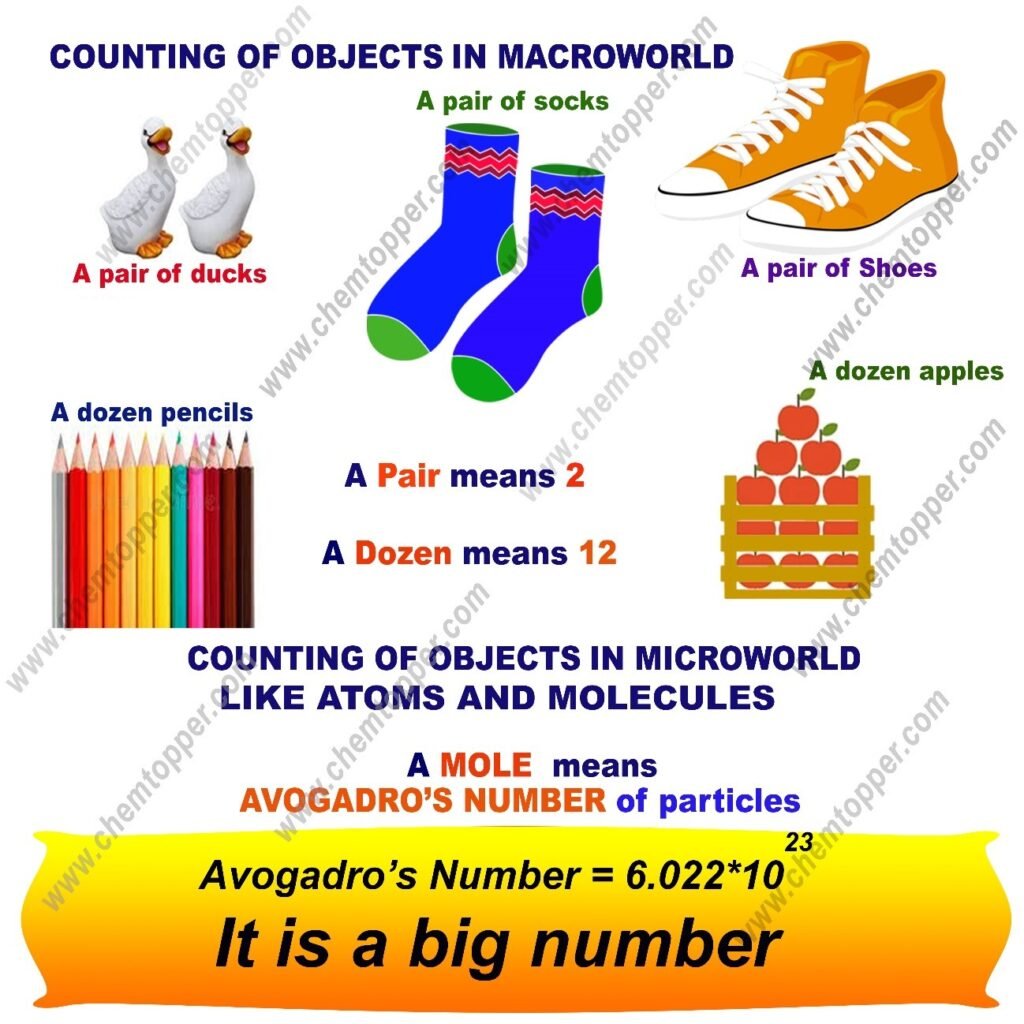
Meaning of a pair represents 2 numbers and meaning of a dozen which represents 12 numbers and meaning of Avogadro’s number which represents a mole or So mole is a counting unit just like a dozen or a pair
Practice Problem
How many water molecules are there ?
In a dozen of water molecules?
Hide- 12 water molecules.
In a pair of water moecules ?
Hide- 2 water molecules
In a mole of water molecules?
Hide- water molecules
Mole is a counting unit
Definition of a mole
A mole (mol) is the amount of a substance that contains same number of particles as there are atoms in exactly 12 g of carbon-12.
So mole is a counting unit just like a dozen or a pair
Number of atoms in 12 g carbon -12 (isotope)
= Avogadro’s number
= 6.022 141 79 × 1023.
Definition of Avogadro’s number
Avogadro’s number is 6.022 141 79 × 1023 number of particles in exactly one mole of any pure substance.
opAvogadro’s Number can be rounded off to 6.022 × 1023.
Avogadro’s number is so large that if all people on the earth start counting atoms in one mole of an element at the rate of 1atom per second, it will take 3 million years for all the atoms to be counted.
Conversion of a mole to number to number of atoms
So, it means
Now Answer this question
Practice Question -How many atoms are there is 50. moles of Calcium atoms ?
Answer (hidden)
Number of atoms of Ca =
=3.0atoms of Calcium
Practice Question -How many moles are there in atoms of Sodium ?
Answer -Hide
Number of atoms of Sodium =
=10.0 moles of Sodium

Molar Mass
The mass of one mole of a pure substance in grams is called as the molar mass of that substance.
Molar mass
Units of molar mass = grams /mole or g/mol
By definition a mole of carbon-12 atoms has a mass of exactly 12 g and this is the molar mass of carbon.
Similarly, 1mole of Oxygen atoms has a mass of exactly 16.00 g and it is the molar mass of Oxygen.
You can use periodic table to know the molar masses of the elements.
Average Atomic masses in grams of an element in the periodic table is the molar mass of the element.
Check for Ag – its average atomic mass is mentioned as 107.87g
So molar mass of Ag is 107.87 g/mol
You can use periodic table to know the molar masses molecules and formula units of ionic compounds.
Let us try to understand the meaning of a formula unit -Mg(NO3)2
has
| One formula unit Mg(NO3)2 | One mol formula unit Mg(NO3)2 | ||
| No of atoms | Number of atoms x atomic masses in u | No of moles of atoms | Atomic masses in grams |
| 1 atom of Mg | 1 x 24.31 | 1 mol of atoms of Mg | 1 x 24.31 |
| 2 atoms of N | 2 x 14.01 | 2 mol of atoms of N | 2 x 14.01 |
| 6 atoms of O | 6 x 16.00 | 6 mol of atoms of O | 6 x 16.00 |
| Mass of 1 unit Mg(NO3)2 | 148.33 u | Mass of 1 mol of Mg(NO3)2 | 148.33 g/mol |
Practice problem -Fill the following table
| One molecule of sugar C12H22O11 | One mole of sugar C12H22O11 | ||
| No of atoms | Number of atoms x atomic masses in u | No of moles of atoms | Atomic masses in grams |
| Mass of 1 molecule C12H22O11 | Mass of 1 mol of C12H22O11 |
Hide answer
| One molecule of sugar C12H22O11 | One mole of sugar C12H22O11 | ||
| No of atoms | Number of atoms x atomic masses in u | No of moles of atoms | Atomic masses in grams |
| 12 atoms of C | 12 x 12.0 | 12 moles of atoms of C | 12 x 12.0 |
| 22 atoms of H | 22 x 1.01 | 22 moles of atoms of H | 22 x 1.01 |
| 11 atoms of O | 11 x 16.00 | 11 moles of atoms of O | 11 x 16.00 |
| Mass of 1 molecule C12H22O11 | 342.22 u | Mass of 1 mol of C12H22O11 | 342.22 g/mol |
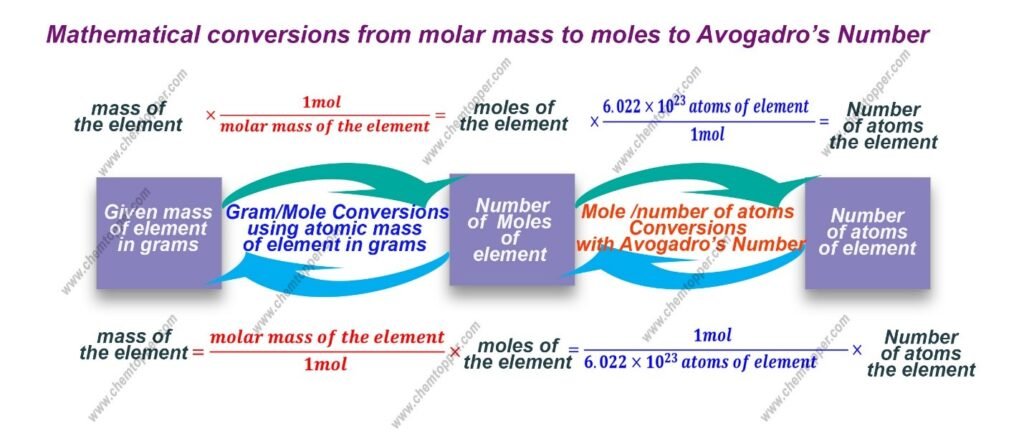
Conversion of moles/grams
Use this ratio
1mol = molar of the substance
Use periodic table to calculate molar mass as explained above.
Mole to grams
Practice problem
How much mass of water is there in 10 moles of water?
Answer
= 180 g
Grams to mole
Grams to mole
How many moles are there is 200 g of CaCO3
Answer
= 10 moles
Conversion of moles/grams with molar mass / number of atoms with Avogadro’s Number
From grams to number of atoms or molecules of the substance
Number of
of the substance
Practice problem
Calculate the number of molecules in 440 grams of CO2
Answer
Number of
of the substance
Practice problem
Calculate the number of molecules in 440 grams of CO2
Answer
From number of atoms or molecules of the substance to grams of the substance
Practice problem :Calculate mass of molecules of Sugar molecules.
Answer
Practice problem :Calculate mass of molecules of Sugar molecules.
Answer
click to copy shortcode